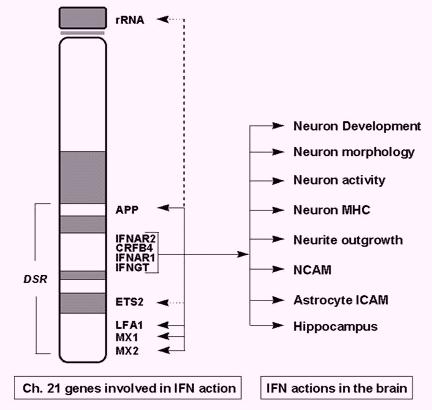
Figure 1
Numerous genes involved in interferon action are located in the region of human chromosome 21. These genes have many dramatic effects on the brain. This figure is excerpted from L.E. Maroun, J. Theor. Biol. 181, 41-46 (1996).
|
Meiogen Biotechnology Corporation P.O. Box 1238 Springfield, IL 62705-1238 (217) 787-5184 Fax: (217) 787-5185 |
Reprinted with the permission of Leonard E. Maroun President and CEO |

In addition to the gene mapping data, a clear correlation is observed for many of the anomalies seen in Down syndrome patient with the side effects seen in patients undergoing interferon therapy.
These are presented in tabular form in Table 1.
| Down Syndrome Anomalies | Interferon Side Effects |
|---|---|
| Mental Retardation2 | Neurotoxicity3, Memory Loss4 |
| frontal lobe dysphasia5 | frontal lobe encephalopathy6 |
| Heart Anomalies7 | Cardiotoxicity8, Arrhythmia9 |
| Leukopenia10 | Leukopenia11 |
| autoimmune disease12 | autoimmune disease13 |
| Hypothyroidism14 | Hypothyroidism15 |
| Hearing Loss16 | Deafness17 |
| Short Stature18 | Growth Inhibition19 |
Table 1
A Comparable Spectrum Of Anomalies Are Seen In Both Down Syndrome And Interferon-Treated Patients.
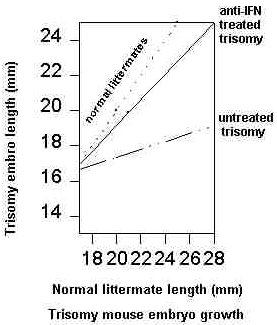
It has been known for some time that the cells from Down syndrome patients are excessively sensitive to the anticellular effects of all interferons (Ref. 20). Recently, a test of the possibility that anti-IFN treatment could help a trisomic individual was completed using a mouse model system. This study supported the prediction that agents which can lower the activity of the interferons could have significant beneficial effects on the developing trisomic mouse (Figure 2, Ref. 21). Meiogen was founded to bring these benefits to the Down syndrome patient.
Although some data suggests that interferon levels are abnormally high in Down syndrome patients, it is more commonly believed that the amount of interferon in the circulation of Down syndrome patients is within normal range. However, because of the presence of extra chromosome 21-coded interferon receptors on the surface of their cells, the Down syndrome patient is supersensitive to interferon and responds to normal levels of interferon as though there were greatly increased interferon levels present. Thus, the goal of treatment is to reduce circulating levels of interferon response back to the normal range. Animal studies where interferon low levels have been effectively reduced to zero by gene deletion procedures indicate that exceptionally low levels of interferon are well tolerated. As would be expected these animals do show an increased susceptibility to infection. Down syndrome patients on therapy will need to be monitored for this possibility.
The Product
There are a number of approaches that could be used to effect a reduction in interferon bioactivity in the Down syndrome patient. Meiogen will use the most rapid and direct approach available by using a soluble form of the human interferon receptor produced as a recombinant (genetically engineered protein ("antiferon™"). Thus, we will mimic in the patient a natural form of regulating the activity of compounds that circulate in the blood. Similar cytokine receptor preparations are already in clinical trials for other diseases (Ref. 22). Test quantities of antiferon™ can be available in a matter of months. By out-sourcing the production and testing of antiferon™ to FDA-approved facilities, Meiogen should be able to complete preclinical testing and submit its first investigational drug application (IND) less than three years from funding.
The Market
There are approximately 500,000 Down syndrome patients in the countries that make up the NAFTA and Common Market Agreements. The number of Down syndrome children born in the U.S. is approximately 4,000 per year. Despite the legalization of abortion, the incidence of Down syndrome in the U.S. newborn population continues to increase (Ref. 1).
The Corporation is not aware of any drugs that have been approved by the FDA or are under consideration for approval by the FDA that would be in competition with the Meiogen product, although it is impossible to know if any such drug products might currently be in the product development pipeline. It is expected that Down syndrome patients would need continuous treatment at least throughout their formative years (birth to age 18). Costs, per- year, for therapy with recombinant proteins comparable to antiferon™ are: Pulmozyme, $10,000/year; Neupogen, $16,000/year; Epogen, $11,000/year; Growth Hormone, $11,000/year (Sources: Biotechnology 13(9), 952 (1995) [Pulmozyme]; Genetic Engineering News 15(12), 27 (1995) [Neupogen and Epogen]; Biotechnology 13(10), 1038 (1995) [Growth Hormone]).
The Clinical Trials
The Down syndrome patient begins life with brain capability (IQ) close to that of the normal child
(Figure 3, Ref 2). These parameters begin to deteriorate until at age 13 Down syndrome patients
display a mean IQ below 50. Thus, the initial target population for treatment will be the Down
syndrome patient from newborn to adolescent. It is expected that these patients will require
continuous treatment, at least throughout the formative years in a manner similar to the way the
diabetic patient receives periodic intramuscular injections of insulin. Treatment efficacy will be
determined using a battery of tests that, depending on the age of the patient, would include the
evaluation of developmental milestones, IQ testing, brain stem and auditory evoked potentials, and
immune system function. Down syndrome patients have well-documented pathologies in each of
these measures.
The target size for the initial study would be thirty Down syndrome placebo controls and thirty treated Down syndrome patients between 4 and 6 years of age with moderate mental retardation and moderate endocrine, cardiac, and infective disease history.
The patients would receive intramuscular injections twice each month for a period of 18 months. Evoked potentials and immunology testing will be performed once every three months. The time of blood drawing and evoked potential activity recording would need to be staggered to avoid association. The actual study size and final design will depend on a number of factors including consideration of statistical power, patient recruitment and retention, and FDA recommendations.
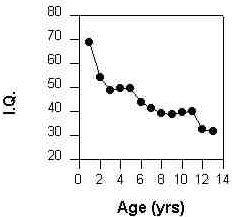
Figure 3
IQ of the Down Syndrome child ages 1 to 13 (Carr, 1985)
Non-Technical Summary
Down syndrome patients are unusually sensitive to the actions of a protein called interferon. Interferon is produced by the cells in our body in response to foreign invaders, like viruses and bacteria. It acts by slowing the growth of cells and promoting early cell death. Currently, interferon is used as a treatment for certain types of cancer. Patients being injected with interferon as a treatment for cancer show side effects, like problems with memory and thyroid function, that are very similar to the medical problems seen in the people with Down syndrome. In addition, there are a number of different genes on Chromosome 21 that are involved in the action of interferon causing the cells of a person with Down syndrome to be exceptionally susceptible to growth retardation and early cell death. Thus, we believe interferon is causing premature death of the brain cells of people with Down syndrome and may be causing some or all of the other symptoms of Down syndrome.
Meiogen is developing a product named "antiferon™" using a technique called "genetic engineering". It is expected that antiferon™ will act like a sponge absorbing and removing the interferon. If interferon is causing some of the symptoms of Down syndrome, then there is a good possibility that injecting the antiferon™ will at least partially correct some of the Down syndrome associated medical problems. Antiferon™ works much like the body's natural way of preventing excessive interferon activity in the body. Experiments in animal models and rare mutations in humans that effectively eliminate their ability to respond to interferon suggest that antiferon™ treatment should be quite safe. However, the only way to determine if antiferon™ treatment will be both safe and effective is to run well-designed clinical trials. These trials will begin as soon as possible, but only after extensive testing of antiferon™ in animals to meet the safety standards and requirements of the U.S. Food and Drug Administration (FDA).
Given adequate funding, the whole process of product development and testing to FDA standards is expected to take about 3 years. The clinical trials go through at least three phases, and each phase should take about two years. Progression to the next phase depends on the success of the previous trial and FDA approval.
At this time, Meiogen's limited goal is to successfully run the early phase (phase I/II) trials. The later trials become successively larger and more expensive and our ability to run these trials will depend on whether or not we are successful in demonstrating that antiferon™ treatment is both safe and effective.
Our early trials will involve about 60 children with Down syndrome randomly divided into treated and untreated groups. The treatment will likely involve injecting antiferon™ about once every two weeks for about 18 months. During this time, we will be looking for improvement in the brain, heart and immune system functions.
The Literature
| Source: http://www.meiogen.com | |
| Revised: November 6, 1998. |